Abstract
Background: Chemorefractory diffuse large B-cell lymphoma (DLBCL) is associated with poor outcomes. Recent Car-T therapy trials, including Zuma-1 which led to the first FDA approval of Car-T for DLBCL (Neelapu NEJM), have shown sustained complete remission, disease control, and long-term survival in a proportion of patients. As with all trials, results must be interepreted in context of study definitions and eligibility parameters. While selection bias is often discussed, little published data regarding specific eligibility requirements on accrual of DLBCL trials exists. To better understand factors influencing Car-T trial eligibility in DLBCL, and context for observed survival rates in Car-T trials, we examined chemorefractory DLBCL patients seen from 2011-2015 at our center, applying key eligibility criteria from Zuma-1 and describing likely reasons for trial exclusion.
Methods: Pts with DLBCL seen at our institution from 2011-2015, who had received at least 2 lines of therapy, were reviewed under IRB approval to determine chemorefractory status based on ZUma-1 definition, and potential eligibility for the Zuma-1 trial. Chemorefractory status per Zuma 1 was defined as stable disease (lasting 6 months or less) or progressive disease as best response to most recent chemotherapy, or disease progression within 12 months of autologous stem cell transplant. "Chemorefractory date" was identified by the chart reviewed, based on biopsy or imaging showing progression, and served as the reference date for reviewing potential Zuma-1 eligibility in detail. Specifically, clinical data (ECOG, labs, organ function) within 8 weeks of chemorefractory date was examined to estimate potential Zuma-1 eligibility. On occasion, more remote studies (e.g., an echocardiogram >8 weeks prior) were applied when data appeared relevant. Descriptive statistics and a Kaplan-Meier survival estimate were performed, with a comparison between those potentially Zuma-1 eligible and those not. No attempt was made to compare outcomes among pts receiving specific therapies for chemorefractory DLBCL.
The specific eligibility factors examined were: histology (DLBCL, PMBCL and tFL); prior therapy including history of allogeneic SCT; CNS involvement, performance status (ECOG 01- vs 2 or higher), laboratory parameters, cardiac disease, infectious comorbidities; history of second malignancy other than nonmelanoma skin cancer/in situ cance or FL; need for urgent therapy due to tumor mass effect or rapid progression.
Results: Of 404 in our DLBCL database from 2011-2015, 163 had received at least 2 therapies and were examined. 36 had inadequate follow up, leaving 127 for detailed analysis.
Of these 127, 78 were determined chemorefractory as per Zuma-1.
Of these 78 chemorefractory pts, median age was 63 (18-82), 17 had transformed lymphoma, 30 underwent transplant (20 auto, 2 allo, 8 auto-allo), 18 relapsed within 1 year of autologous transplant, and 30 had primary refractory disease.
43 patients (55%) were deemed ineligible for Zuma-1 by retrospective review, for reasons given in Table 1.
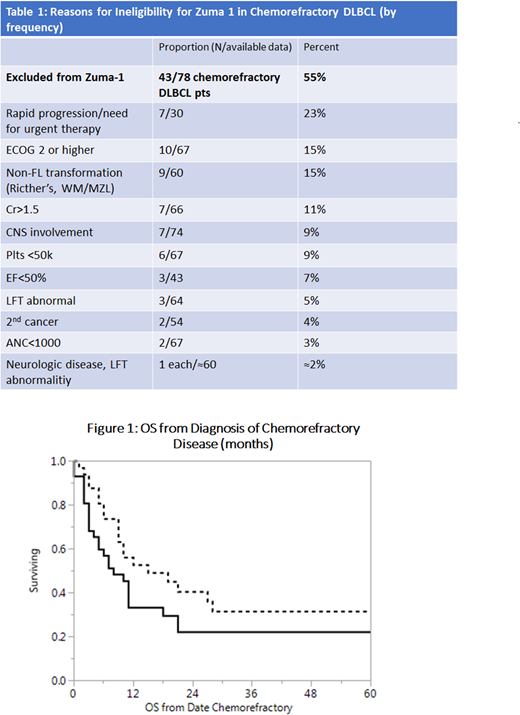
Figure 1 shows survival. Among "eligible" pts vs not: Median OS was 15 vs 8 months (eligible vs not, p=.04). 1 yr OS was 56% vs 33%, and 2 yrs OS 40% vs 22%.
Conclusion: When applied to a historical cohort, about half of chemorefractory DLBCL pts met eligibility criteria for Zuma-1. The survival of "eligible" patients appears significantly better than others. A need for acute therapy (for rapid progression), ECOG performance status 2 or greater, and non-FL transformation (Richter's/CLL history) were the most common reasons for exclusion. Since these three features may not impact safety of Car-T therapy, but are associated with agrgessive disease, broadening eligibility around these criteria could represent a step toward testing Car-T therapies among those with greatest unmet need.
Lynch:T.G. Therapeutics: Research Funding; Rhizen Pharmaceuticals S.A.: Research Funding; Incyte Corporation: Research Funding; Takeda Pharmaceuticals: Research Funding; Johnson Graffe Keay Moniz & Wick LLP: Consultancy. Shadman:TG Therapeutics: Research Funding; AstraZeneca: Consultancy; Genentech: Research Funding; Verastem: Consultancy; Mustang Biopharma: Research Funding; Celgene: Research Funding; Gilead Sciences: Research Funding; AbbVie: Consultancy; Qilu Puget Sound Biotherapeutics: Consultancy; Acerta Pharma: Research Funding; Pharmacyclics: Research Funding; Genentech: Consultancy; Beigene: Research Funding. Till:Mustang Bio: Patents & Royalties, Research Funding. Shustov:SPECTRUM PHARMACEUTICALS: Consultancy, Research Funding. Gopal:Janssen: Consultancy, Research Funding; Asana: Consultancy; Takeda: Research Funding; Merck: Research Funding; BMS: Research Funding; Spectrum: Research Funding; Teva: Research Funding; Pfizer: Research Funding; Seattle Genetics: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Brim: Consultancy; Aptevo: Consultancy; Incyte: Consultancy. Smith:Pharmacyclics: Research Funding; Genentech: Research Funding; Acerta Pharma BV: Research Funding; Incyte Corporation: Research Funding; Merck Sharp and Dohme Corp.: Consultancy, Research Funding; Portola Pharmaceuticals: Research Funding; Seattle Genetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal